Umdlavuza wamaphaphu ongewona omncane (i-NSCLC) ubala cishe ama-80% -85% yenani eliphelele lomdlavuza wamaphaphu, futhi ukuhlinza kabusha kuyindlela ephumelela kakhulu yokwelashwa okukhulu kwe-NSCLC yokuqala. Kodwa-ke, ngokuncipha kwe-15% kuphela kokuphindaphinda kanye nokwenza ngcono okungu-5% kokusinda kweminyaka emi-5 ngemuva kokwelashwa ngamakhemikhali okuhlinzekwe ngasikhathi sinye, kunesidingo esikhulu somtholampilo esingahlangatshezwana naye.
I-Perioperative immunotherapy ye-NSCLC iyindawo entsha yocwaningo eminyakeni yamuva nje, futhi imiphumela yenombolo yesigaba sesi-3 sokuhlola okulawulwa ngokungahleliwe isungule isikhundla esibalulekile se-perioperative immunotherapy.
I-Immunotherapy yeziguli ezinomdlavuza wamaphaphu ongewona omncane weseli (i-NSCLC) yenze inqubekelaphambili ebalulekile eminyakeni yamuva nje, futhi lelisu lokwelapha aligcini nje ngokunweba ukusinda kweziguli, kodwa futhi lithuthukisa izinga lempilo, lihlinzeka ngesengezo esisebenzayo ekuhlinzekweni kwendabuko.
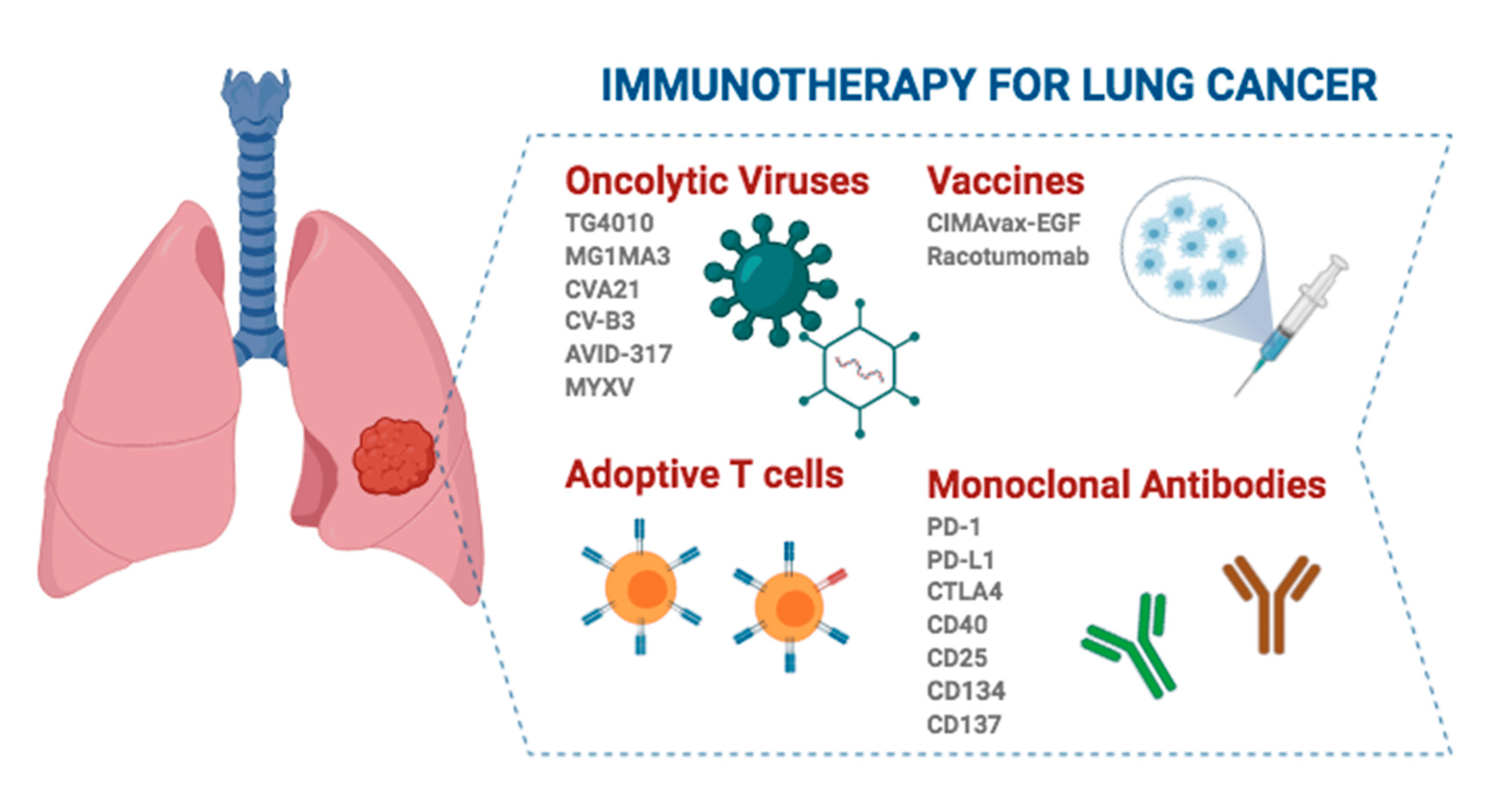
Kuye ngokuthi i-immunotherapy iphathwa nini, kunamaphethini amathathu ayinhloko e-immunotherapy ekwelapheni kwe-NSCLC yesigaba sokuqala esisebenzayo:
1. I-Neoadjuvant immunotherapy iyodwa: I-Immunotherapy yenziwa ngaphambi kokuhlinzwa ukuze kuncishiswe ubukhulu besimila futhi kunciphise ingozi yokuphinda. Ucwaningo lwe-CheckMate 816 [1] lubonise ukuthi i-immunotherapy ehlanganiswe ne-chemotherapy ithuthukise kakhulu ukusinda ngaphandle kwemicimbi (EFS) esigabeni se-neoadjuvant uma kuqhathaniswa nokwelashwa ngamakhemikhali kuphela. Ukwengeza, i-neoadjuvant immunotherapy nayo inganciphisa izinga lokuphindaphinda ngenkathi ithuthukisa izinga lokuphendula eliphelele le-pathological (pCR) yeziguli, ngaleyo ndlela yehlise amathuba okuphindaphinda kwangemva kokuhlinzwa.
2. I-Perioperative immunotherapy (i-neoadjuvant + i-adjuvant) : Kule modi, i-immunotherapy ilawulwa ngaphambi nangemva kokuhlinzwa ukuze kwandiswe umphumela wayo we-antitumor futhi iqhubeke nokususa izilonda ezincane ezisele ngemva kokuhlinzwa. Umgomo oyinhloko wale modeli yokwelapha uwukuthuthukisa ukuphila kwesikhathi eside nokwelapha amazinga eziguli zesimila ngokuhlanganisa i-immunotherapy ezigabeni ze-neoadjuvant (zangaphambi kokusebenza) kanye ne-adjuvant (post-operative). Inothi elingukhiye 671 limele le modeli [2]. Njengokuphela kwesivivinyo esilawulwa ngokungahleliwe (RCT) esine-EFS enhle kanye namaphoyinti okugcina we-OS, sihlole ukusebenza kahle kwe-palizumab kuhlanganiswe nokwelashwa ngamakhemikhali esigabeni esitholakala kabusha ngezikhathi ezithile Ⅱ, ⅢA, kanye ne-ⅢB (N2) iziguli ze-NSCLC. Uma kuqhathaniswa nokwelashwa ngamakhemikhali kuphela, i-pembrolizumab ehlangene nokwelashwa ngamakhemikhali yandisa i-EFS emaphakathi ngeminyaka engu-2.5 futhi yanciphisa ingozi yokuqhubeka kwesifo, ukuphindaphinda, noma ukufa ngama-41%; I-KEYNOTE-671 iphinde ibe wucwaningo lokuqala lwe-immunotherapy ukukhombisa inzuzo yokusinda iyonke (OS) ku-NSCLC ephindayo, ngokuncipha okungama-28% engcupheni yokufa (HR, 0.72), okuyingqophamlando ku-neoadjuvant kanye ne-adjuvant immunotherapy ye-NSCLC esebenzayo yesigaba sokuqala.
3. I-Adjuvant immunotherapy yodwa: Kule modi, iziguli azizange zithole ukwelashwa kwezidakamizwa ngaphambi kokuhlinzwa, futhi ama-immunodrugs asetshenziswa ngemva kokuhlinzwa ukuvimbela ukuphindaphinda kwezicubu ezisele, ezifanele iziguli ezinengozi enkulu yokuphindaphinda. Ucwaningo lwe-IMpower010 luhlole ukusebenza kahle kwe-postoperative adjuvant attilizumab ngokumelene nokwelashwa okuphelele okusekela ezigulini ezinesigaba esikhishwe ngokuphelele se-IB kuya ku-IIA (i-AJCC 7th edition) NSCLC [3]. Imiphumela yabonisa ukuthi ukwelashwa okuhambisanayo ne-attilizumab kwandisa kakhulu ukusinda okungenasifo (i-DFS) ezigulini ezine-PD-L1 ezine-positive esigabeni ⅱto ⅢA. Ngaphezu kwalokho, ucwaningo lwe-KEYNOTE-091/PEARLS luhlole umphumela we-pembrolizumab njengokwelashwa okungeziwe ezigulini ezibuyiselwe ngokuphelele ngesigaba IB kuya ku-IIA NSCLC [4]. I-Pabolizumab inwetshwe ngokuphawulekayo enanini labantu lilonke (HR, 0.76), ne-DFS emaphakathi yezinyanga ezingama-53.6 eqenjini le-Pabolizumab nezinyanga ezingama-42 eqenjini le-placebo. Eqenjini elincane leziguli ezine-PD-L1 tumor proportion score (TPS) ≥50%, nakuba i-DFS yandiswa isikhathi eside eqenjini le-Pabolizumab, umehluko phakathi kwamaqembu amabili awubalulekile ngokwezibalo ngenxa yobukhulu besampula obuncane, futhi ukulandelwa okude kwakudingeka ukuze kuqinisekiswe.
Ngokusho kokuthi i-immunotherapy ihlanganiswe nezinye izidakamizwa noma izinyathelo zokwelapha kanye nemodi yokuhlanganisa, uhlelo lwe-neoadjuvant immunotherapy kanye ne-adjuvant immunotherapy lungahlukaniswa ngezindlela ezintathu eziyinhloko ezilandelayo:
1. I-Single immunotherapy: Lolu hlobo lokwelapha luhlanganisa izifundo ezifana ne-LCMC3 [5], IMpower010 [3], KEYNOTE-091/PEARLS [4], BR.31 [6], kanye ne-ANVIL [7], ebonakala ngokusetshenziswa kwezidakamizwa ze-immunotherapy njenge-adjuvant therapy (entsha).
2. Inhlanganisela ye-immunotherapy kanye ne-chemotherapy: Izifundo ezinjalo zihlanganisa i-KEYNOTE-671 [2], i-CheckMate 77T [8], i-AEGEAN [9], i-RATIONALE-315 [10], i-Neotorch [11], ne-IMpower030 [12]. Lezi zifundo zibheke imiphumela yokuhlanganisa i-immunotherapy kanye ne-chemotherapy esikhathini sokusebenza.
3. Inhlanganisela ye-immunotherapy nezinye izindlela zokwelapha: (1) Ukuhlanganiswa namanye ama-immunodrugs: Isibonelo, i-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) ihlanganiswe ekuhlolweni kwe-NEOSTAR [13], i-lymphocyte activation gene 3 (LAG-3) i-antibody yahlanganiswa ku-NEO, i-immunoglobulin kanye ne-Test 1 cell Izakhiwo ze-ITIM zahlanganiswa ekuhlolweni kwe-SKYSCRAPER 15 Izifundo ezifana nenhlanganisela ye-TIGIT antibody [15] ziye zathuthukisa umphumela wokulwa nesimila ngokuhlanganiswa kwezidakamizwa zokuzivikela ezifweni. (2) Ihlanganiswe ne-radiotherapy: isibonelo, i-duvaliumab ehlanganiswe ne-stereotactic radiotherapy (SBRT) iklanyelwe ukuthuthukisa umphumela wokwelapha we-NSCLC yokuqala [16]; (3) Inhlanganisela nezidakamizwa ezilwa ne-angiogenic: Isibonelo, ucwaningo lwe- EAST ENERGY [17] luhlole umphumela we-synergistic we-ramumab kuhlanganiswe ne-immunotherapy. Ukuhlolwa kwezindlela eziningi ze-immunotherapy kubonisa ukuthi indlela yokusetshenziswa kwe-immunotherapy esikhathini sokuhlinzwa ayikaqondwa ngokugcwele. Nakuba i-immunotherapy iyodwa ibonise imiphumela emihle ekwelashweni kwe-perioperative, ngokuhlanganisa i-chemotherapy, i-radiation therapy, i-antiangiogenic therapy, namanye ama-immune checkpoint inhibitors afana ne-CTLA-4, i-LAG-3, ne-TIGIT, abacwaningi banethemba lokuqhubeka nokuthuthukisa ukusebenza kahle kwe-immunotherapy.
Asikabikho isiphetho ngemodi elungile yokwelashwa kwe-immunotherapy ye-NSCLC yokuqala esebenzayo, ikakhulukazi ukuthi i-perioperative immunotherapy uma iqhathaniswa ne-neoadjuvant immunotherapy iyodwa, nokuthi ingabe i-adjuvant immunotherapy eyengeziwe ingaletha imiphumela ebalulekile eyengeziwe, kusenokuntuleka kwemiphumela yokuhlolwa eqondile yokuqhathanisa.
Forde et al. kusetshenziswe ukuhlaziya okulinganiselwe kwesilinganiso sesilinganiso sokuhlola umphumela wokuhlola okulawulwa ngokungahleliwe, futhi kwalungisa izibalo zabantu eziyisisekelo nezici zesifo phakathi kwezibalo ezihlukene zocwaningo ukuze kuncishiswe umphumela odidayo walezi zici, okwenza imiphumela ye-CheckMate 816 [1] kanye ne-CheckMate 77T [8] iqhathanise kakhudlwana. Isikhathi sokulandelela esimaphakathi sasiyizinyanga ze-29.5 (CheckMate 816) kanye nezinyanga ze-33.3 (CheckMate 77T), ngokulandelana, ukunikeza isikhathi esanele sokulandelela ukuze kugcinwe i-EFS nezinye izinyathelo ezibalulekile zokusebenza.
Ekuhlaziyweni okunesisindo, i-HR ye-EFS yayingu-0.61 (95% CI, 0.39 kuya ku-0.97), ephakamisa ingozi ephansi engu-39% yokuphindaphinda noma ukufa eqenjini le-perioperative nabuliumab elihlangene le-chemotherapy (imodi ye-CheckMate 77T) uma kuqhathaniswa ne-neoadjuvant nabuliumab ehlanganisiwe ye-chemotherapy1 iqoqo le-chemotherapy1. Iqembu le-perioperative nebuliuzumab plus chemotherapy libonise inzuzo enesizotha kuzo zonke iziguli ezisesiteji sokuqala, futhi umphumela wabonakala kakhulu ezigulini ezinenkulumo engaphansi kwe-1% yesimila PD-L1 (ukunciphisa ngama-49% engcupheni yokuphinda noma yokufa). Ukwengeza, ezigulini ezihlulekile ukufeza i-pCR, iqembu le-perioperative nabuliumab elihlangene le-chemotherapy libonise inzuzo enkulu ye-EFS (ukunciphisa ama-35% engozini yokuphindaphinda noma ukufa) kuneqembu le-neoadjuvant nabuliumab elihlangene le-chemotherapy. Le miphumela iphakamisa ukuthi imodeli ye-perioperative immunotherapy inenzuzo kakhulu kunemodeli ye-neoadjuvant immunotherapy iyodwa, ikakhulukazi ezigulini ezinenkulumo ephansi ye-PD-L1 kanye nezinsalela zesimila ngemva kokwelashwa kokuqala.
Kodwa-ke, ezinye iziqhathaniso ezingaqondile (ezifana nokuhlaziywa kwe-meta) azizange zibonise umehluko omkhulu ekusindeni phakathi kwe-neoadjuvant immunotherapy kanye ne-perioperative immunotherapy [18]. Ukuhlaziywa kwe-meta okusekelwe kudatha yesiguli ngasinye kutholwe ukuthi i-perioperative immunotherapy kanye ne-neoadjuvant immunotherapy ibe nemiphumela efanayo ku-EFS kuwo womabili ama-pCR kanye nama-non-PCR subgroups ezigulini ezine-NSCLC yesigaba sokuqala esisebenzayo [19]. Ukwengeza, umnikelo wesigaba se-adjuvant immunotherapy, ikakhulukazi ngemva kokuba iziguli zithole i-pCR, uhlala uyinkinga emtholampilo.
Muva nje, iKomidi Lokweluleka Ngezidakamizwa Lase-US Food and Drug Administration (FDA) lixoxe ngalolu daba, ligcizelela ukuthi indima ethile ye-adjuvant immunotherapy ayikacaci [20]. Kwaxoxwa ngokuthi: (1) Kunzima ukuhlukanisa imiphumela yesigaba ngasinye sokwelashwa: ngoba uhlelo lwe-perioperative luqukethe izigaba ezimbili, i-neoadjuvant kanye ne-adjuvant, kunzima ukucacisa umnikelo ngamunye wesigaba ngasinye emphumeleni jikelele, okwenza kube nzima ukunquma ukuthi yisiphi isigaba esibaluleke kakhulu, noma ngabe zombili izigaba zidinga ukwenziwa ngesikhathi esisodwa; (2) Amathuba okwelashwa ngokweqile: uma i-immunotherapy ihileleke kuzo zombili izigaba zokwelashwa, ingabangela iziguli ukuba zithole ukwelashwa ngokweqile futhi zandise ingozi yemiphumela emibi; (3) Umthwalo wokwelashwa okhulisiwe: Ukwelashwa okwengeziwe esigabeni sokwelashwa kwe-adjuvant kungaholela emthwalweni ophezulu wokwelashwa ezigulini, ikakhulukazi uma kunokungaqiniseki mayelana negalelo lakho ekusebenzeni kahle okuphelele. Ekuphenduleni inkulumo-mpikiswano engenhla, ukuze kufinyelelwe esiphethweni esicacile, izivivinyo eziklanywe ngokuqinile eziklanywe ngokungahleliwe ziyadingeka ukuze kuqinisekiswe okwengeziwe esikhathini esizayo.
Isikhathi sokuthumela: Dec-07-2024